Multiple SonoScape Products Obtained the CE Mark under EU-MDR
1970-01-01 08:33:42
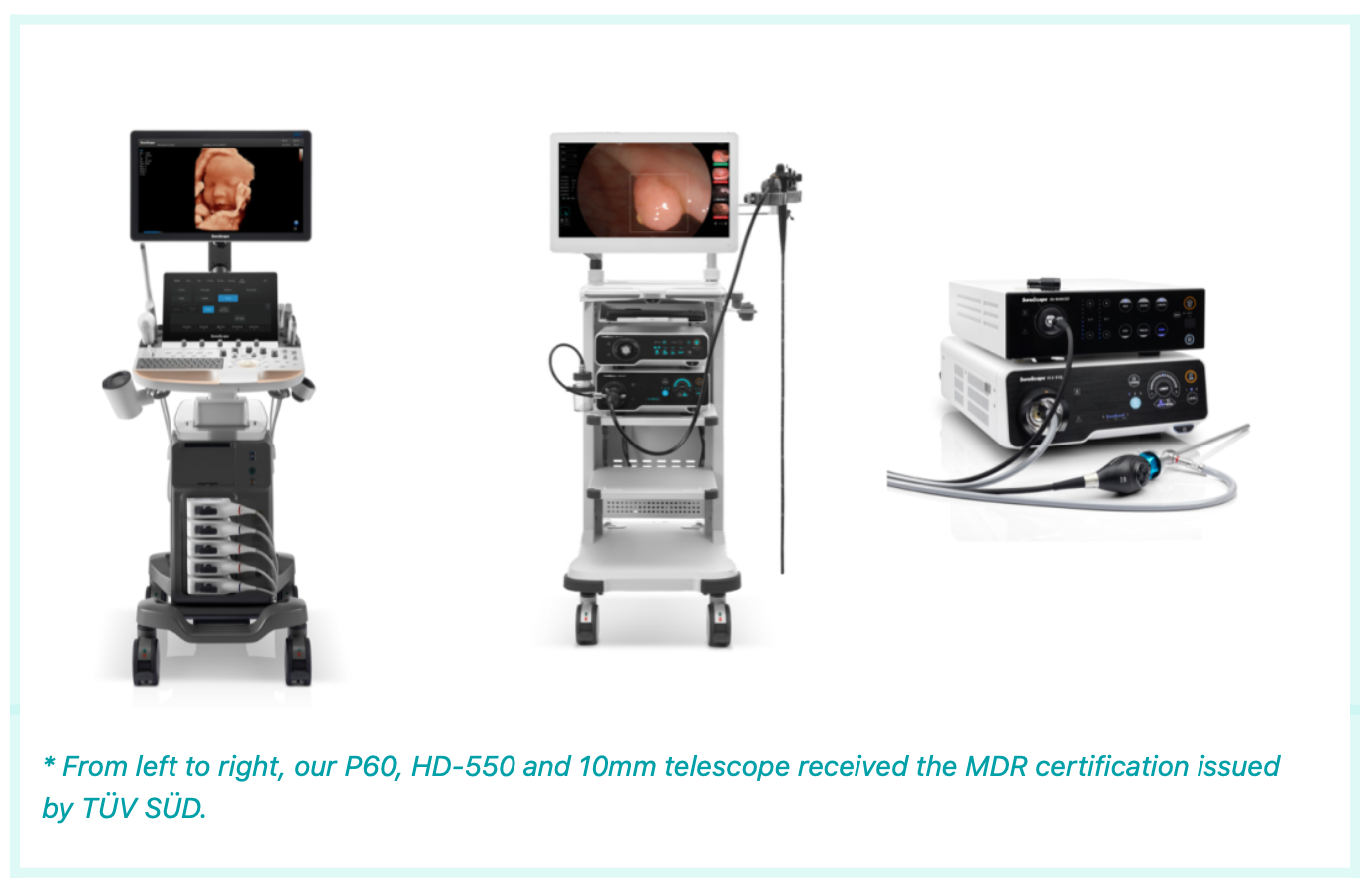
With the commitment to continued innovation, SonoScape offers a comprehensive and expanding portfolio of imaging technologies including solutions for ultrasound, endoscopy, and MIS that strengthens its role as a solution provider to enhance the diagnosis and treatment capability of medical institutions.
In mid-April this year, SonoScape HD-550 received FDA 510(k) clearance, extending its offerings to USA healthcare providers with a brighter way of image clarity. Further CE-MDR approval gives SonoScape a significant impetus to the global commercialization process. "The MDR raises the bar for our medical devices sold in the EU with more strict compliance requirements," said Steven He, Sales Director of the Endoscopy Division. "Receiving the CE mark under the MDR promises our high-level protection of patient safety and our long-term support for the EU endoscopist community.”
“It's extremely exciting to witness SonoScape achieve what it is today, with the EU passport for both its ultrasound and endoscopy system.” David Liao, Sales Director of the Ultrasound Division commented. “We are very pleased to have achieved this milestone and look forward to bringing in the new way of ultrasound diagnosis efficiency to our EU journey ahead.”
